X(suda) + 2Y(suda) → Tuz + Su Yukarıda verilen nötrleşme tepkimesindeki X ve Y maddelerinin formülü aşağıda verilenlerden hangisi olamaz? Dyod ne ne eigns x Y A) Ca(OH)2 CH3COOH B) H2SO4 NaOH

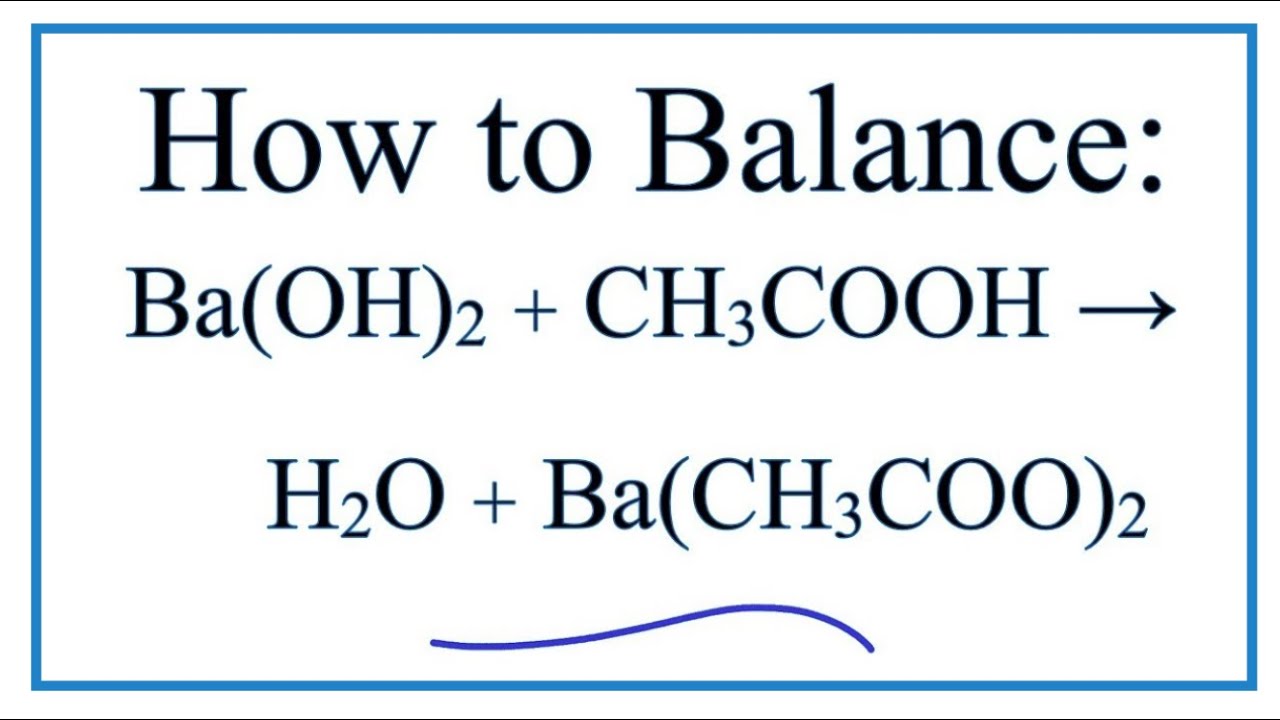
The complete balanced equation for the reaction between barium hydroxide and acetic acid is - brainly.com

100 mL of 0.1 M CH3COOH, 50 mL of 0.1 M HCl is mixed with 50 mL of 0.1 M Ba( OH)2. The pH of mixture of solution is (pK, of CH3COOH is 4.74)

sebanyak 50 mL larutan Ba(OH)2 dapat dinetralkan dengan 20 mL larutan CH3COOH 0,1M. tentukan massa - Brainly.co.id
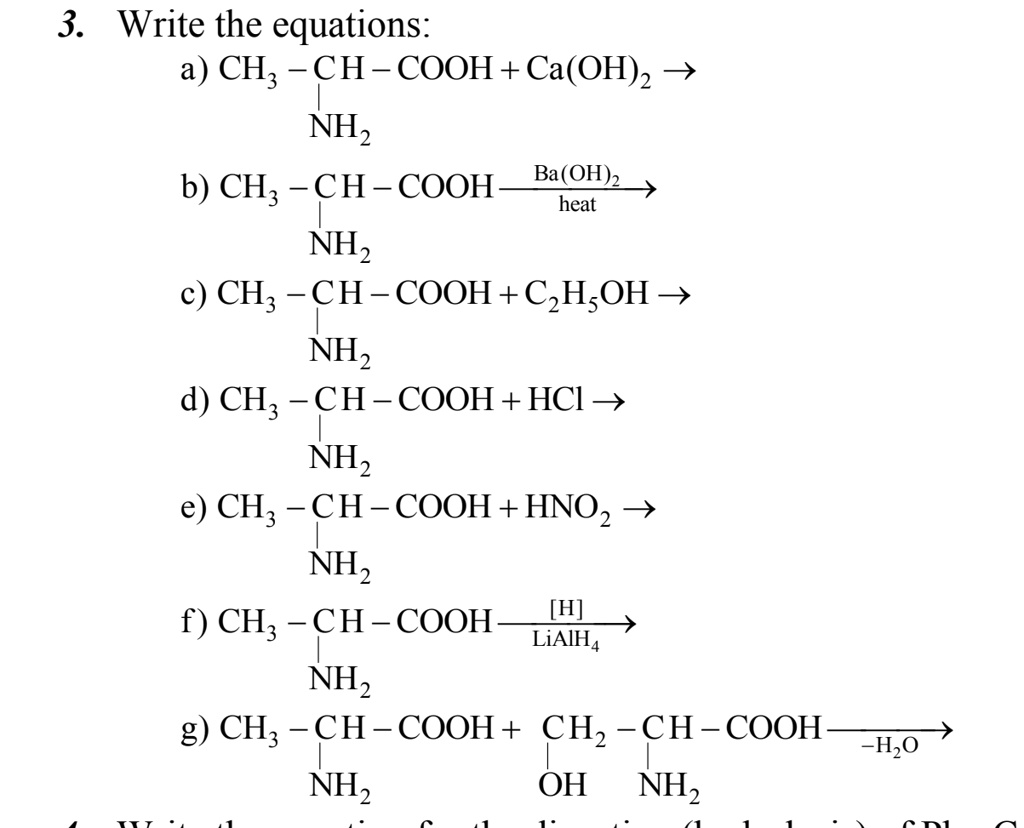
SOLVED: Write the equations: a) CH3COOH + Ca(OH)2 â†' NH2COOH + Ba(OH)2 b) CH3COOH + heat â†' NH2COOH + C2H5OH c) CH3COOH + HCI â†' NH2COOH + H2O d) CH3COOH + HNO2

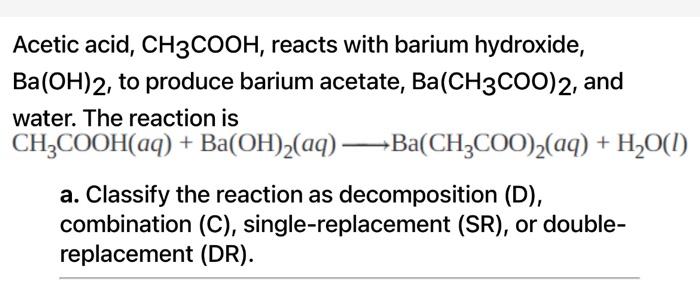
SOLVED: Enter the molecular equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced molecular equation. Identify all of the phases in your answer: CH3COOH(aq) +
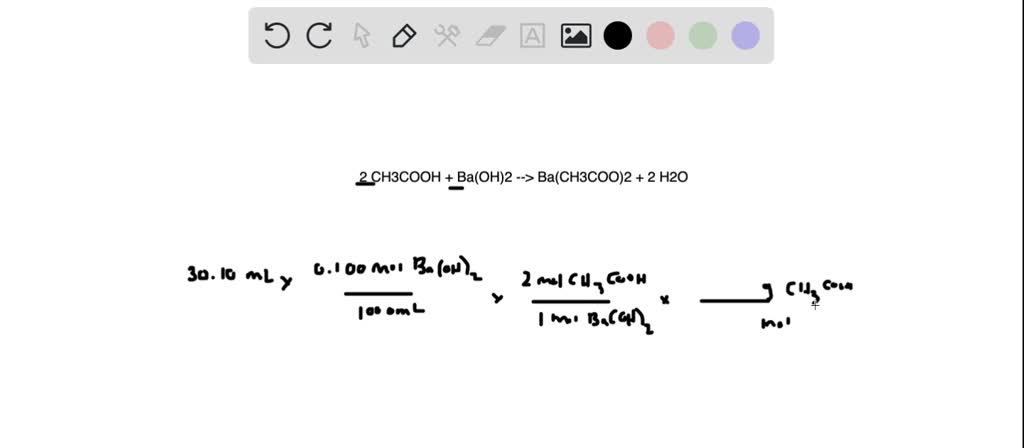
SOLVED: Vinegar is a solution of acetic acid, CH3COOH, dissolved in water. A 5.54-g sample of vinegar was titrated to equivalence point by 30.10 mL of 0.100 M Ba(OH)2 . What is